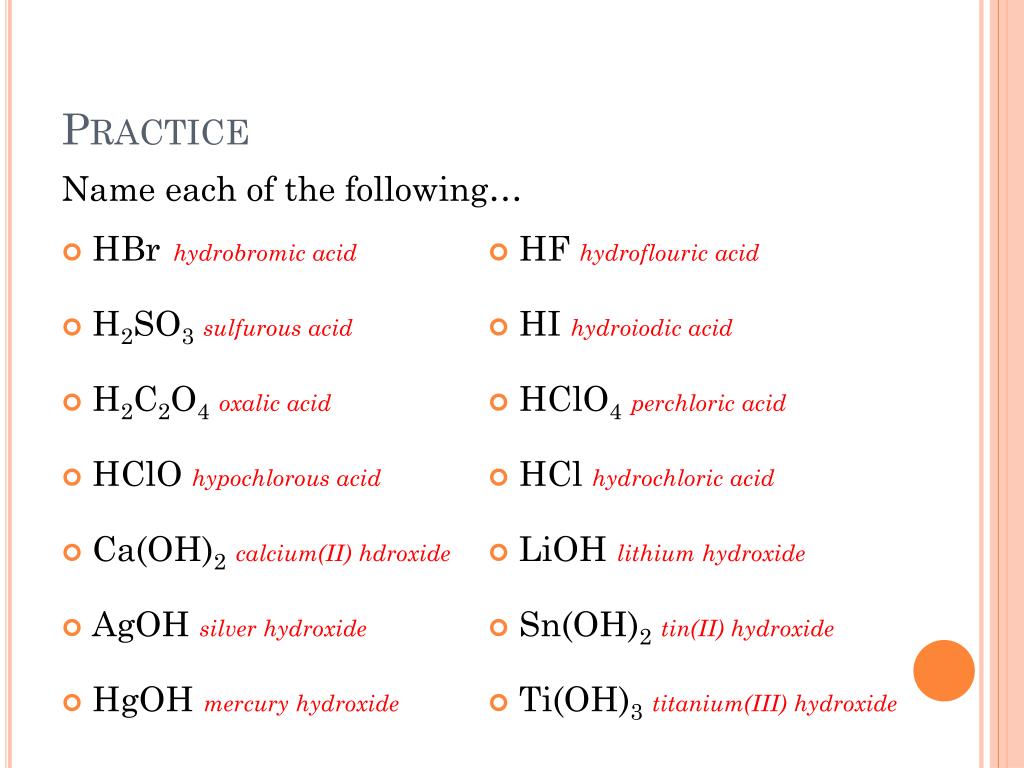
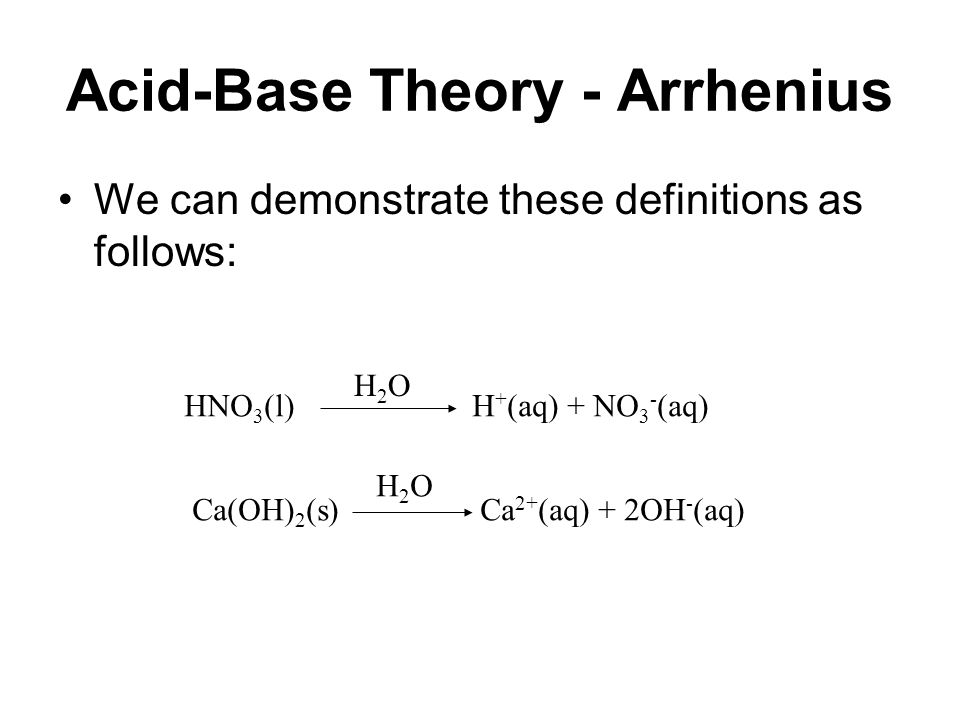
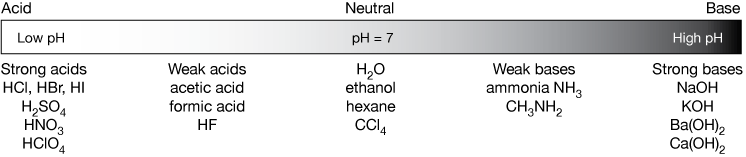
Acid and Base Chemistry. Some Properties of Acids þ Produce H + (as H 3 O + ) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) - ppt download
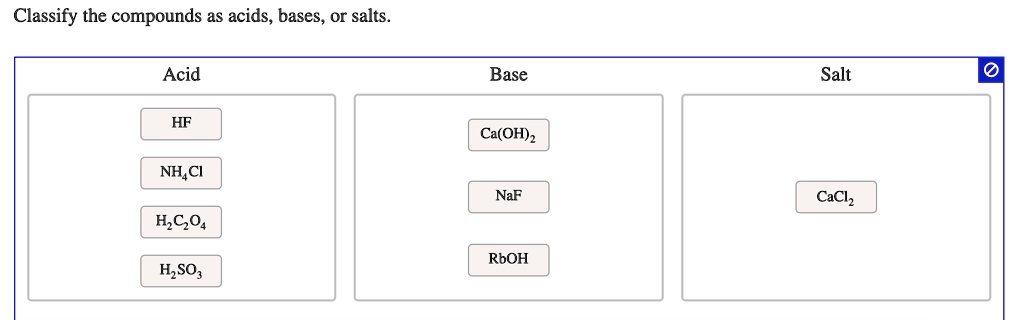
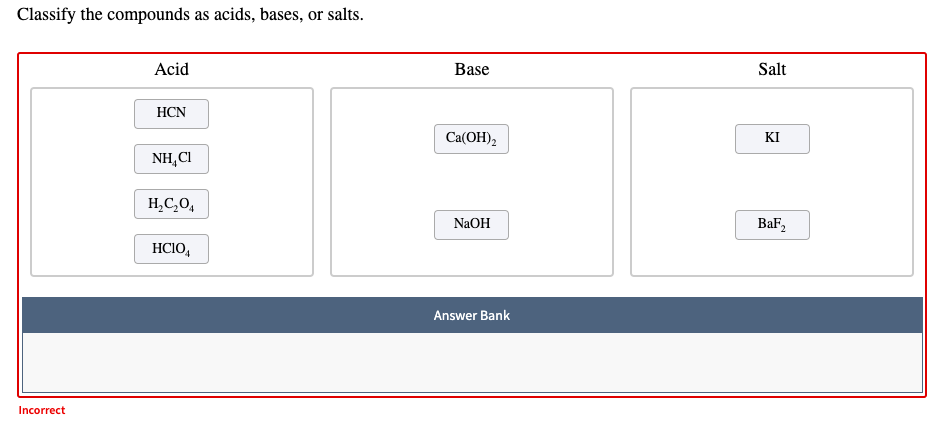
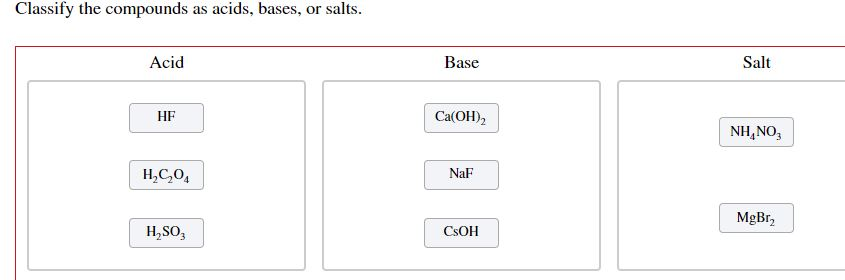
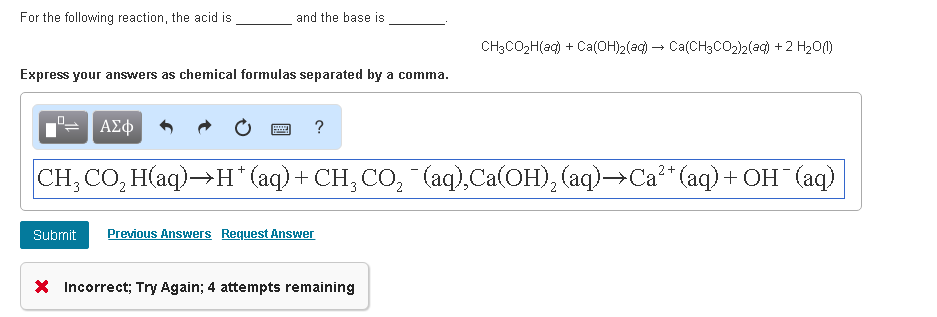
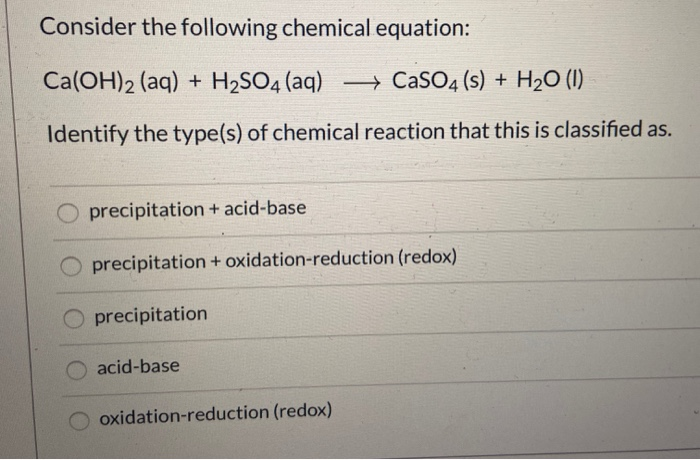
SOLVED: Classify the compounds as acids, bases, or salts Acid Base Salt HF Ca(OH)2 NH,CI NaF HCO4 RbOH HzSO, CaClz
Which of the following reacts is not shown by formic acid? Reaction with Ca( OH)2 Reaction with I2 / Red P Reaction with NaHCO3 Reaction with C2H5OH
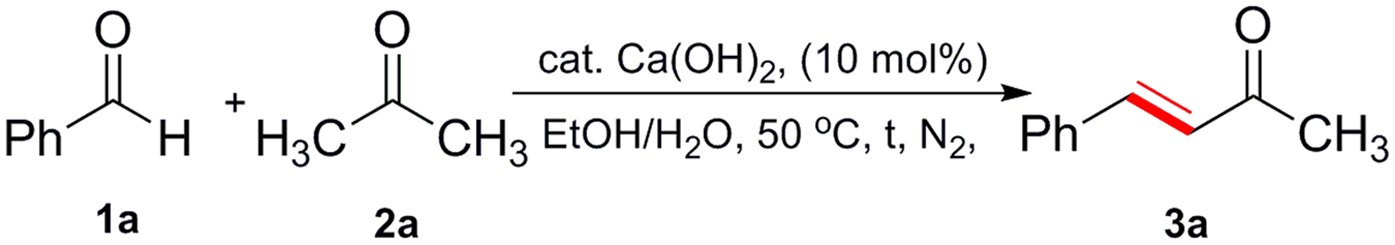
Ca(OH)2-Catalyzed Condensation of Aldehydes with Methyl ketones in Dilute Aqueous Ethanol: A Comprehensive Access to α,β-Unsaturated Ketones | Scientific Reports
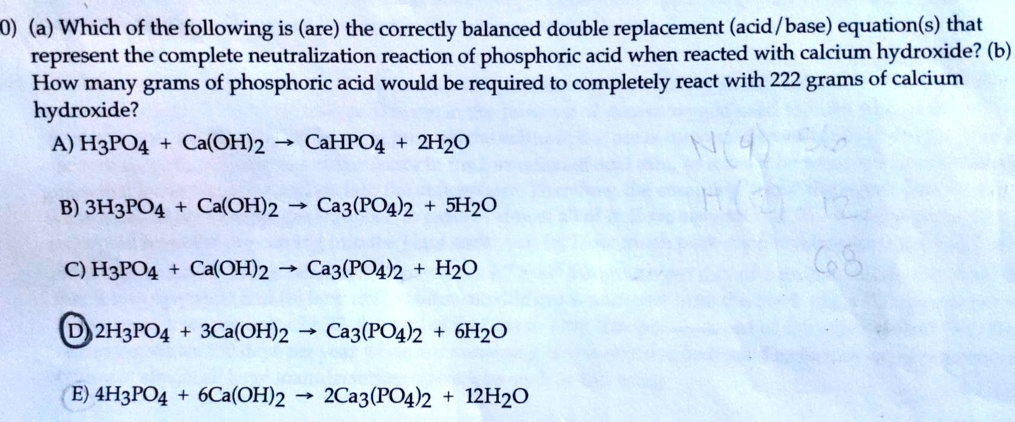
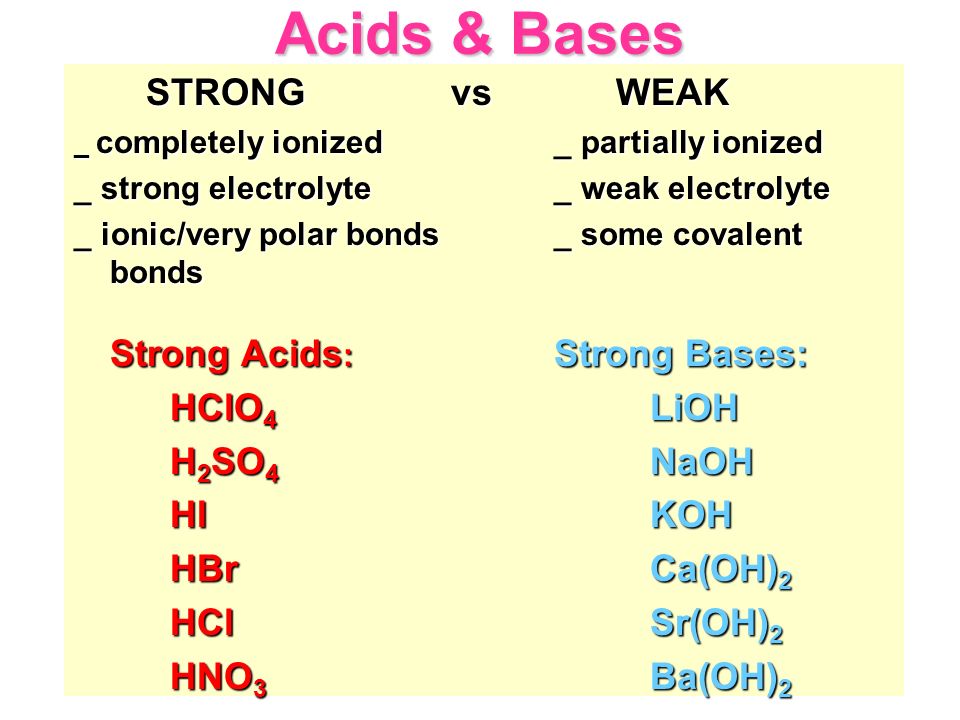



.PNG)