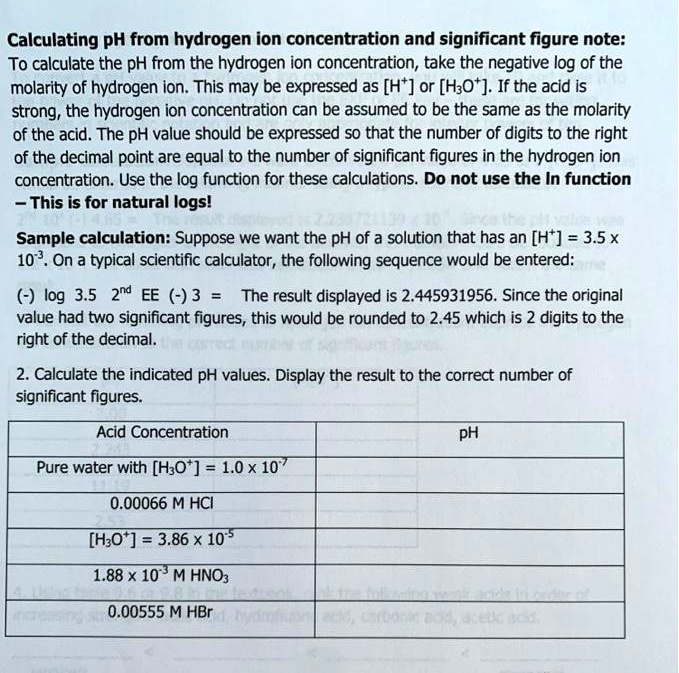
SOLVED: Calculating pH from hydrogen ion concentration and significant figure note: To calculate the pH from the hydrogen ion concentration; take the negative log of the molarity of hydrogen ion. This may

Vidéo de question : Identifier l'équation utilisée pour calculer la concentration en ions hydronium | Nagwa
![SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M](https://cdn.numerade.com/ask_previews/e9f7adf9-a742-4ec8-bdea-5a0f18192bbf_large.jpg)
SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M
![SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have](https://cdn.numerade.com/ask_previews/48330dbe-2875-4cae-b450-b3566371f267_large.jpg)
SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have
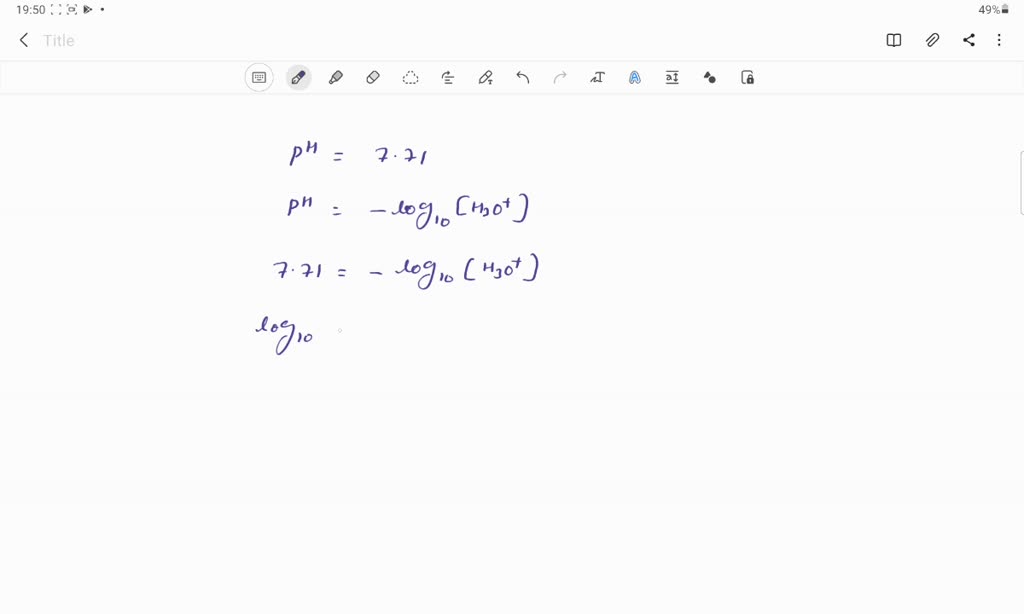
SOLVED: Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 7.710
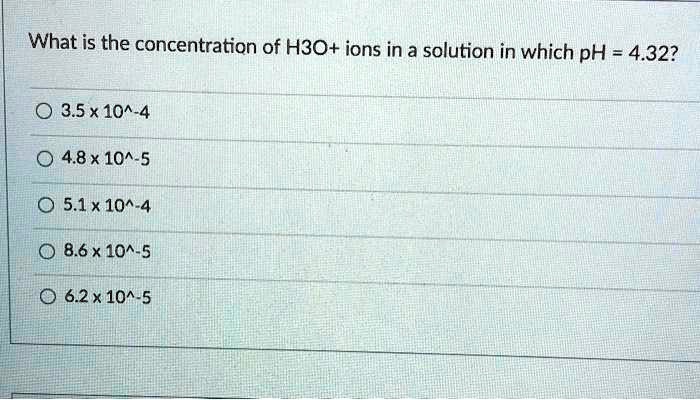
SOLVED: What is the concentration of H3O+ ions in a solution in which pH = 4.32? 3.5x 10^-4 4.8x 10^-5 0 5.1*10^-4 8.6 x 10^-5 6.2*10^-5

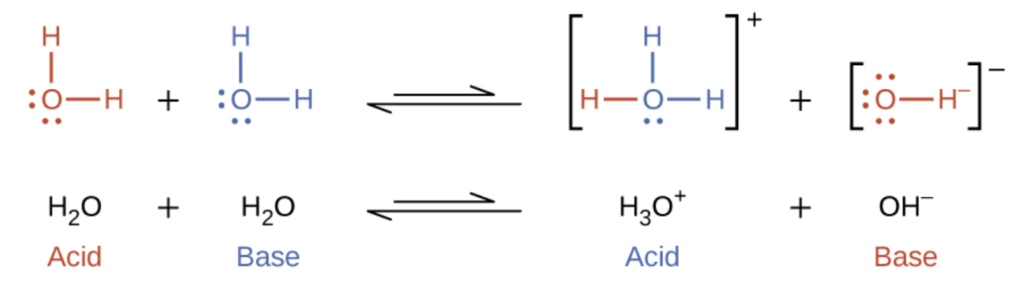
SOLVED: The pH value is an expression of the molarity of H3O+ ions in solution. This concentration has strong impact on chemical reactions. The molarity is very small value, and therefore awkward
SOLVED: 1. What is the pH of a urine sample that has an H3O+ concentration of 1 x 10-5 M? Classify the solution as acidic, basic or neutral. 2. What is the
![SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x](https://cdn.numerade.com/ask_images/6dfe549d4995435d873f5dcfa09e91b7.jpg)
SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x
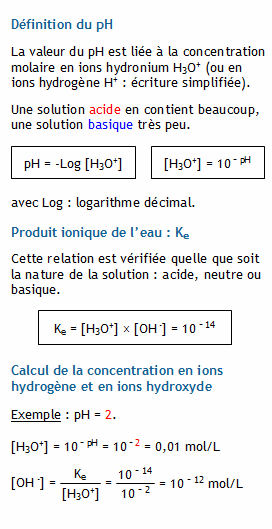
Calculer la concentration molaire en ions hydrogène et en ions hydroxyde d'une solution connaissant son pH

Vidéo de question : Calculer la concentration en ions hydroxyde d'un échantillon à partir de son pH | Nagwa

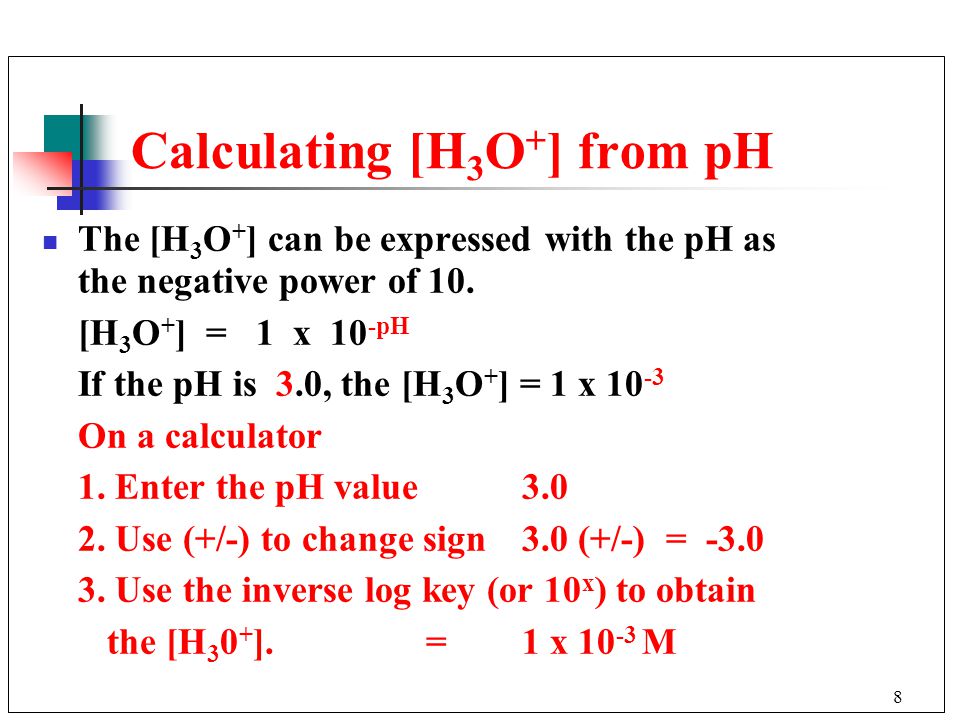





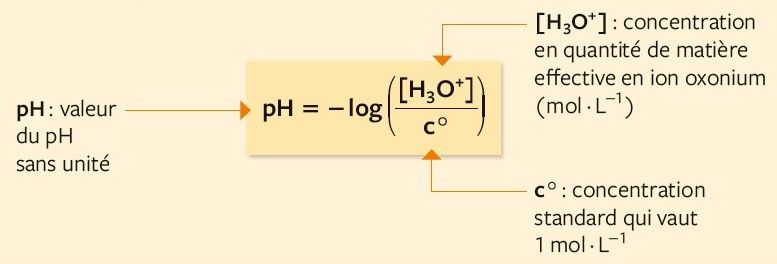

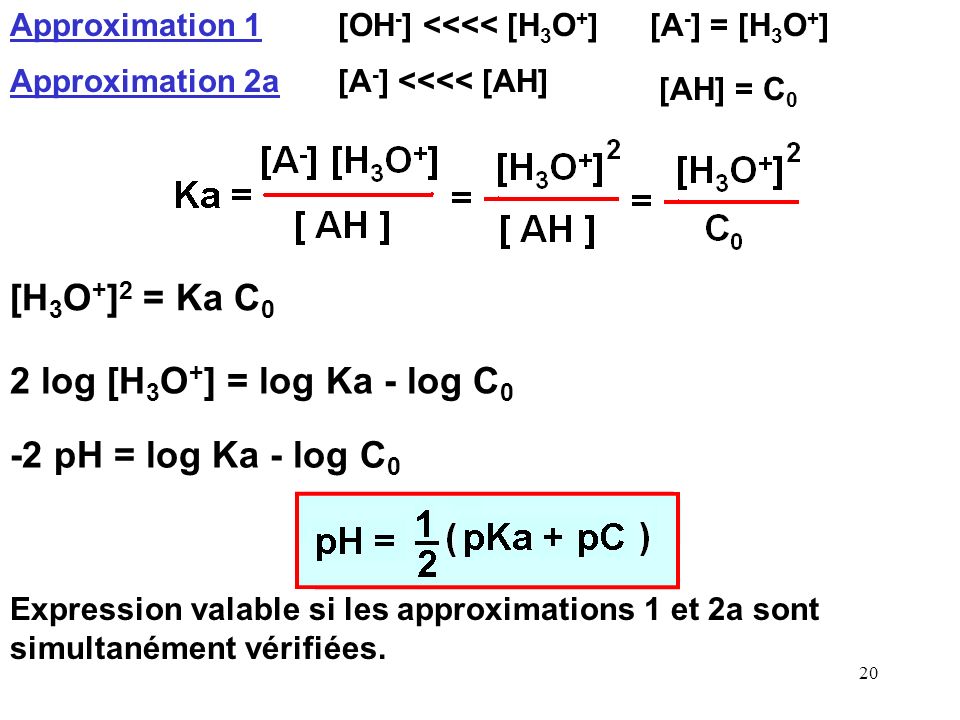



![pH and [H3O+] without a Calculator - YouTube pH and [H3O+] without a Calculator - YouTube](https://i.ytimg.com/vi/H_mst8MqrW8/maxresdefault.jpg)