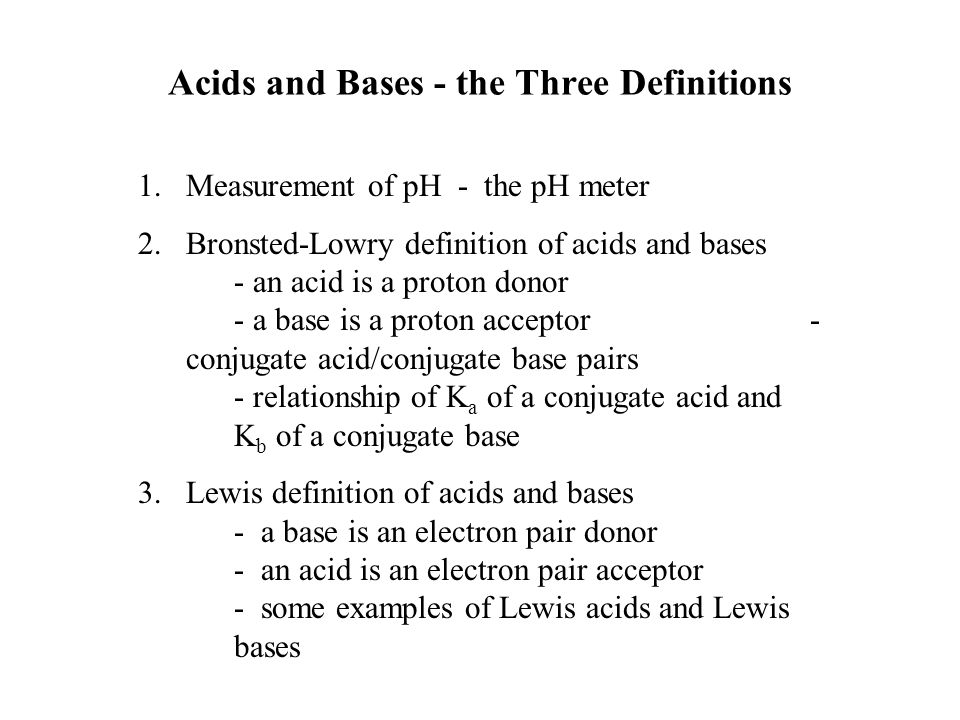
Acids and Bases - the Three Definitions 1.Measurement of pH - the pH meter 2.Bronsted-Lowry definition of acids and bases - an acid is a proton donor - - ppt download
![Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download](https://images.slideplayer.com/20/6056284/slides/slide_2.jpg)
Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download
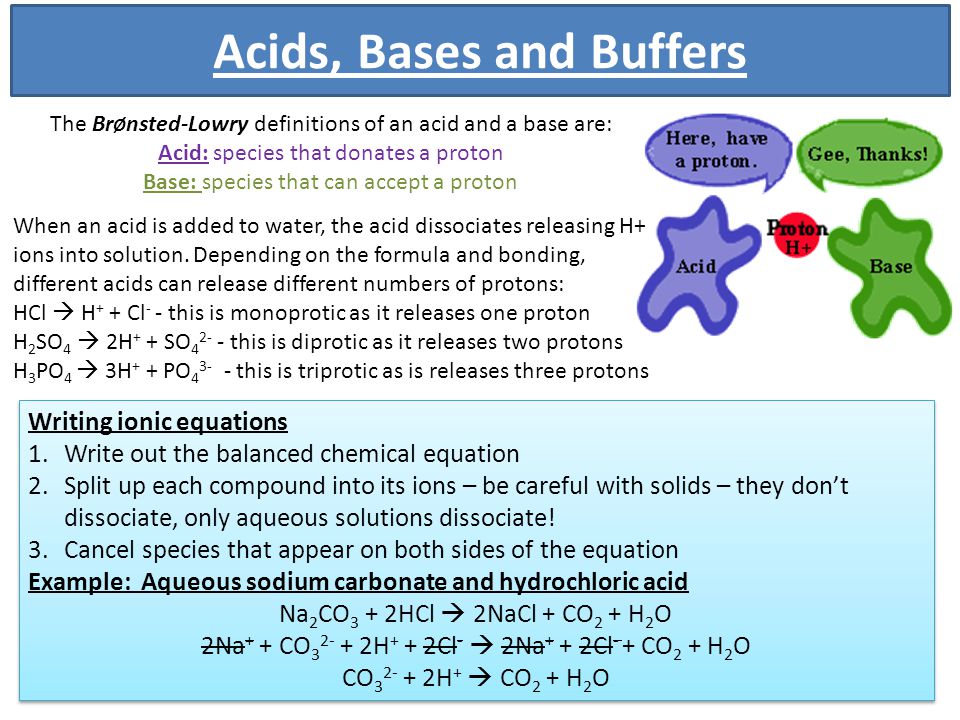
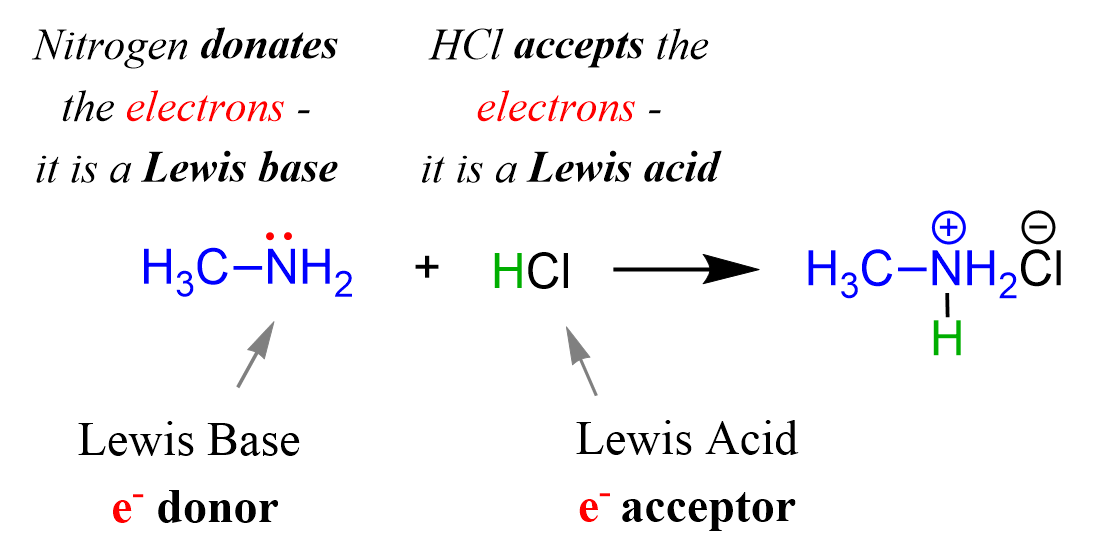
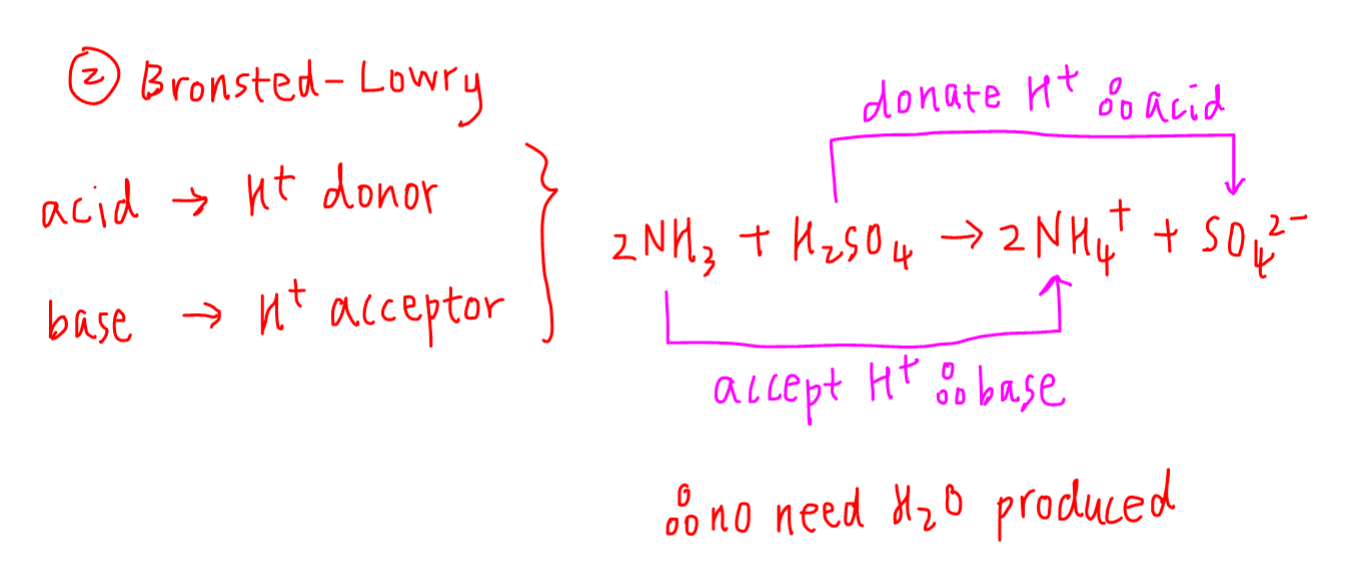
Acids, Bases and Buffers The Br Ø nsted-Lowry definitions of an acid and a base are: Acid: species that donates a proton Base: species that can accept. - ppt download

Lewis Acids and Bases - Definition,Properties, Examples, Reactions, Uses, Applications of Lewis acids and Bases.
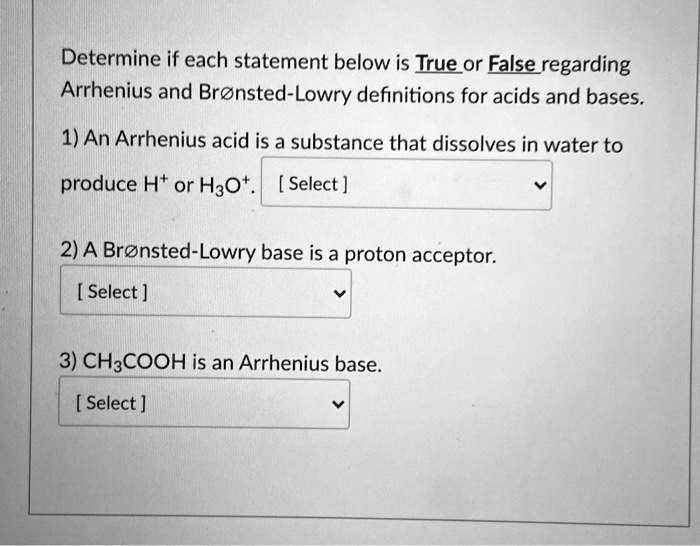
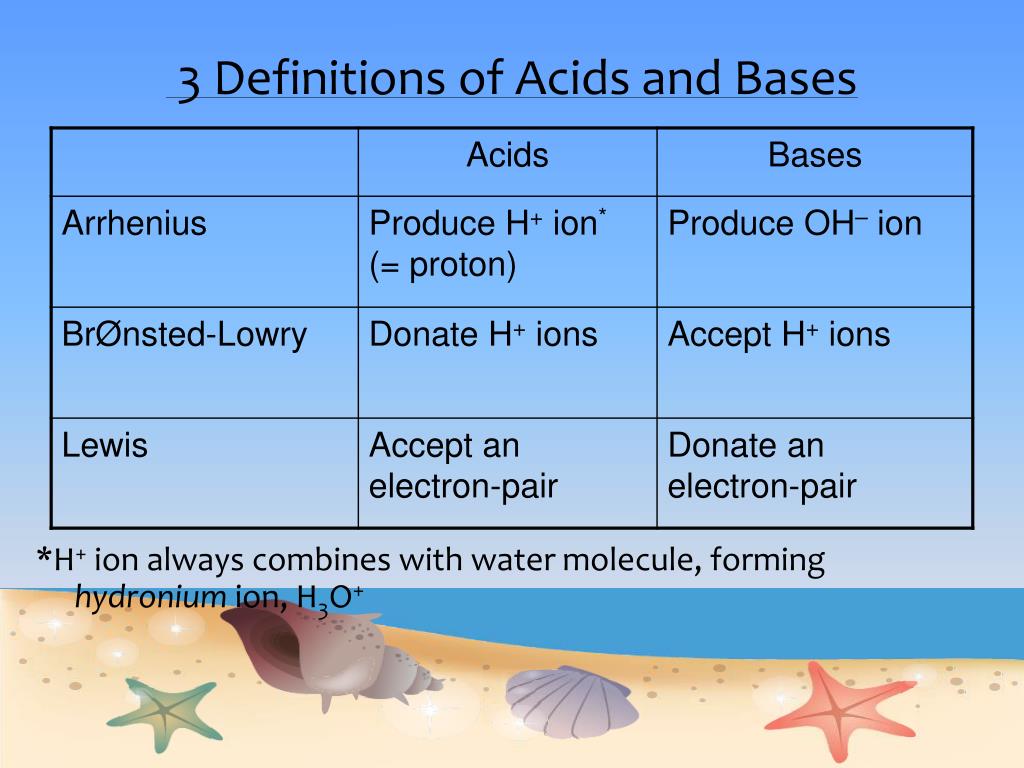
SOLVED: Determine if each statement below is Trueor Ealse regarding Arrhenius and Bronsted-Lowry definitions for acids and bases. 1) An Arrhenius acid is a substance that dissolves in water to produce Ht