n-BuLi as a Highly Efficient Precatalyst for Hydrophosphonylation of Aldehydes and Unactivated Ketones | Organic Letters

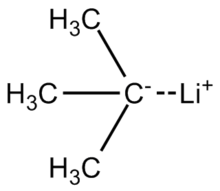
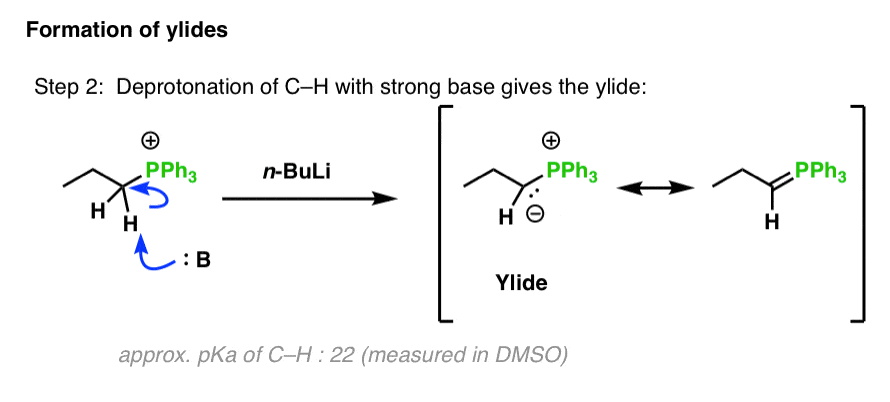
Lithium diisopropylamide is a strong base and nonnucleophilic base. It is often freshly prepared by treating a certain reactant with n-butyllithium (n -BuLi). Draw the starting material and draw the product (lithium diisopropylamide).

n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine-Mediated Ortholithiations of Aryl Oxazolines: Substrate-Dependent Mechanisms | Journal of the American Chemical Society

n‐Butyllithium (1 mol %)‐catalyzed Hydroboration of Aldehydes and Ketones with Pinacolborane - Yang - 2019 - Bulletin of the Korean Chemical Society - Wiley Online Library

n-BuLi/LiCH2CN-Mediated One-Carbon Homologation of Aryl Epoxides into Conjugated Allyl Alcohols | Organic Letters