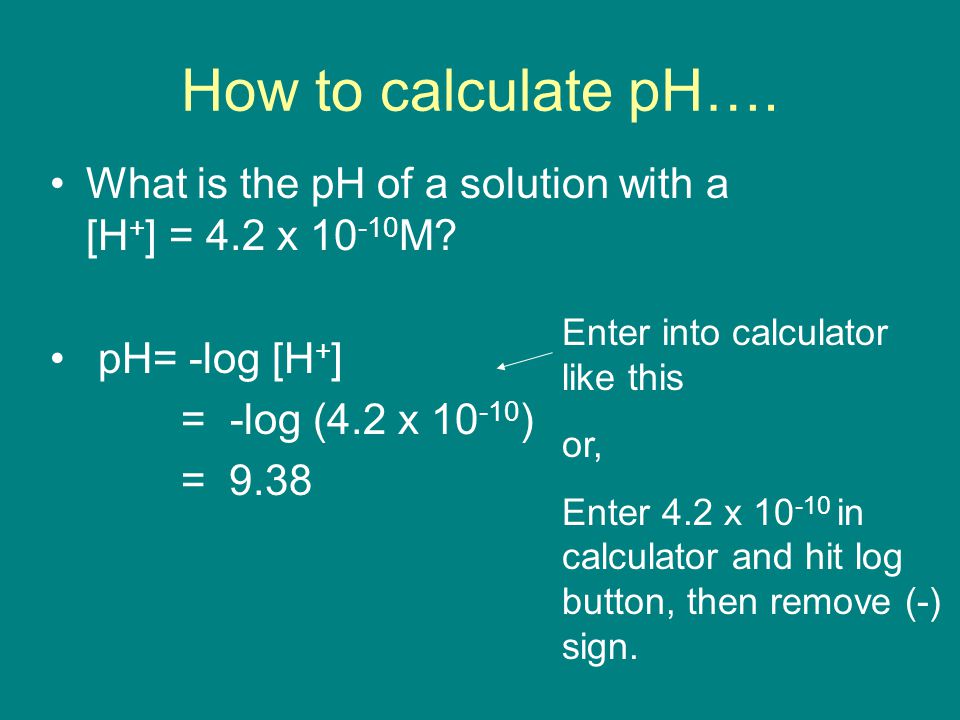
![pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarit… | Chemistry lessons, Chemistry education, Chemistry classroom pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarit… | Chemistry lessons, Chemistry education, Chemistry classroom](https://i.pinimg.com/736x/99/80/05/998005d7b3fbb74f7a91222f3209e7c5--physical-chemistry-ap-chemistry.jpg)
pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarit… | Chemistry lessons, Chemistry education, Chemistry classroom
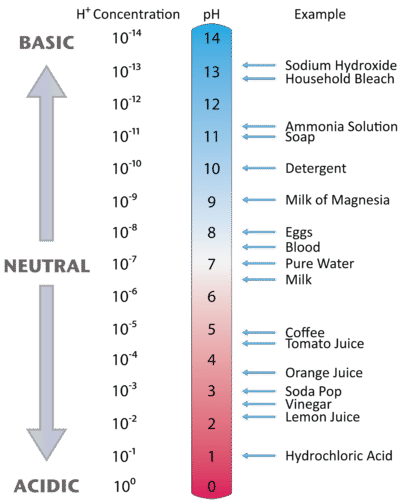
Would a solution with a [H+] concentration of 1 X 10-11 have a pH value of ___ and be considered a(n)? - Quora
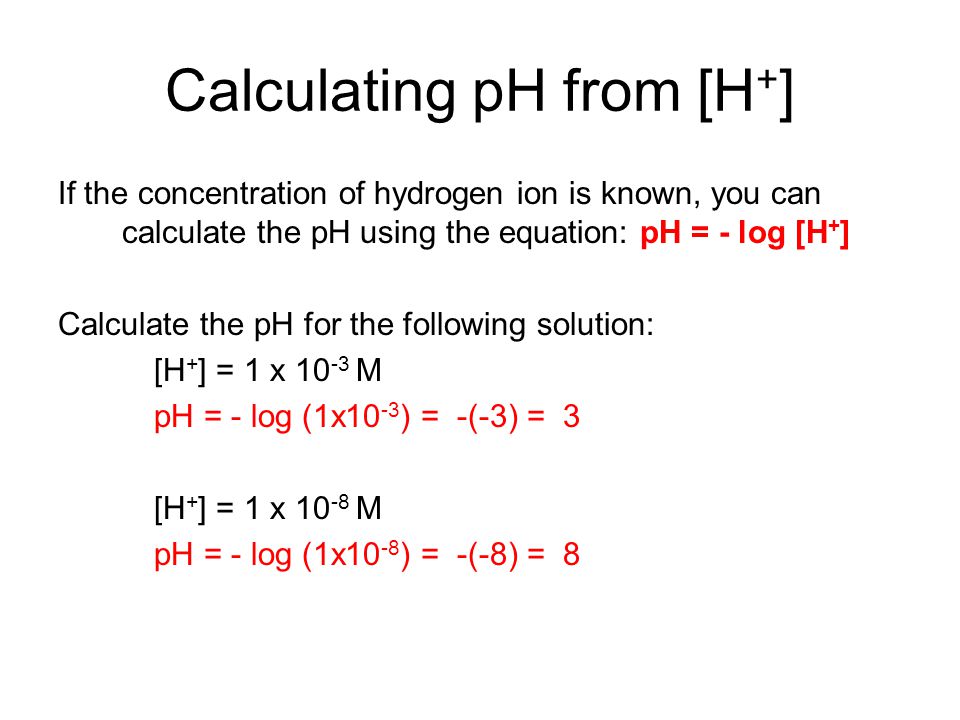

![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)
![Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC" Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC"](http://itsactuallyquitebasic.weebly.com/uploads/2/7/8/0/27808159/4950515.png?357)




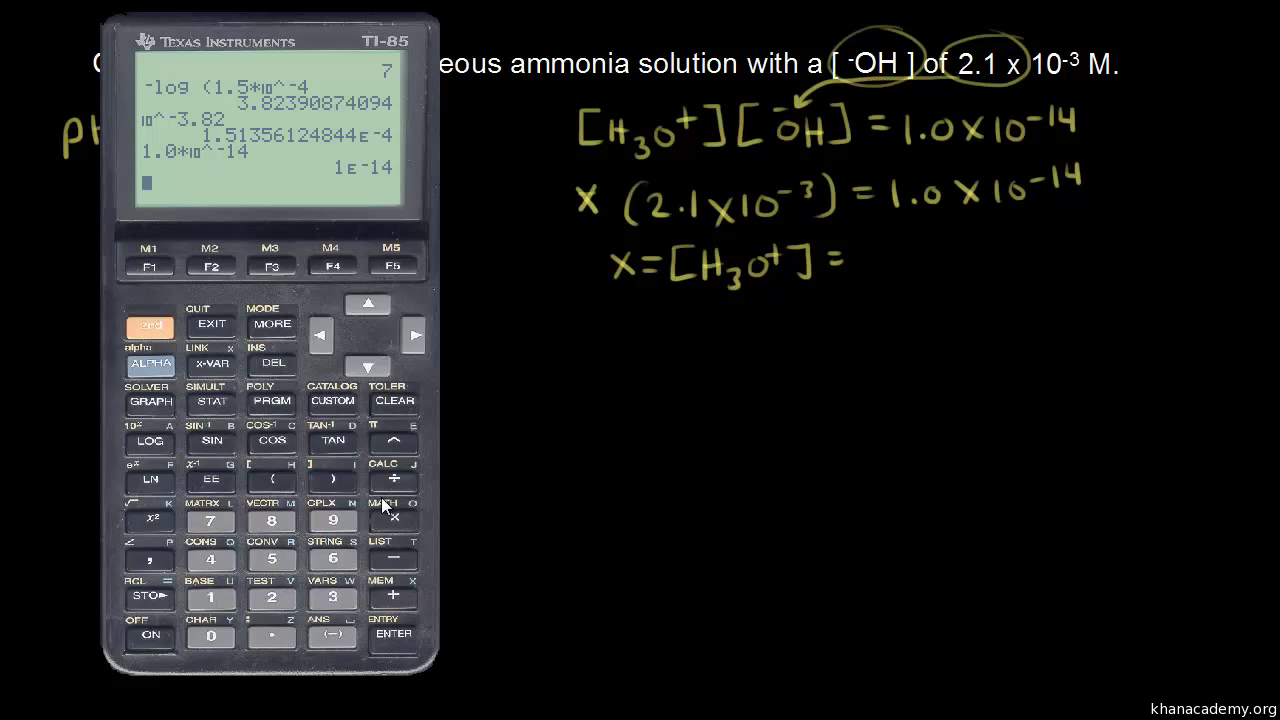
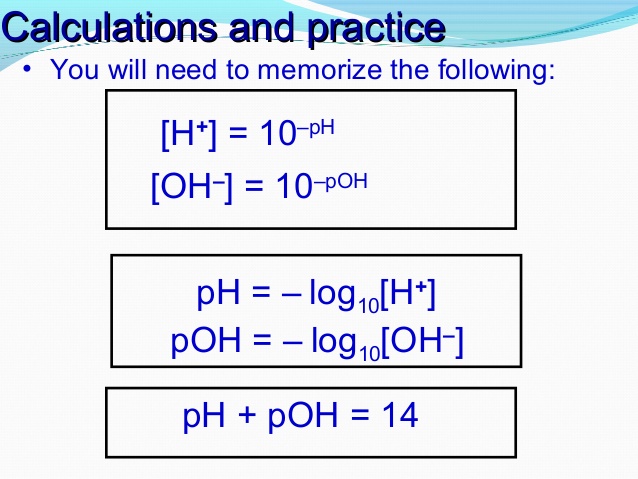

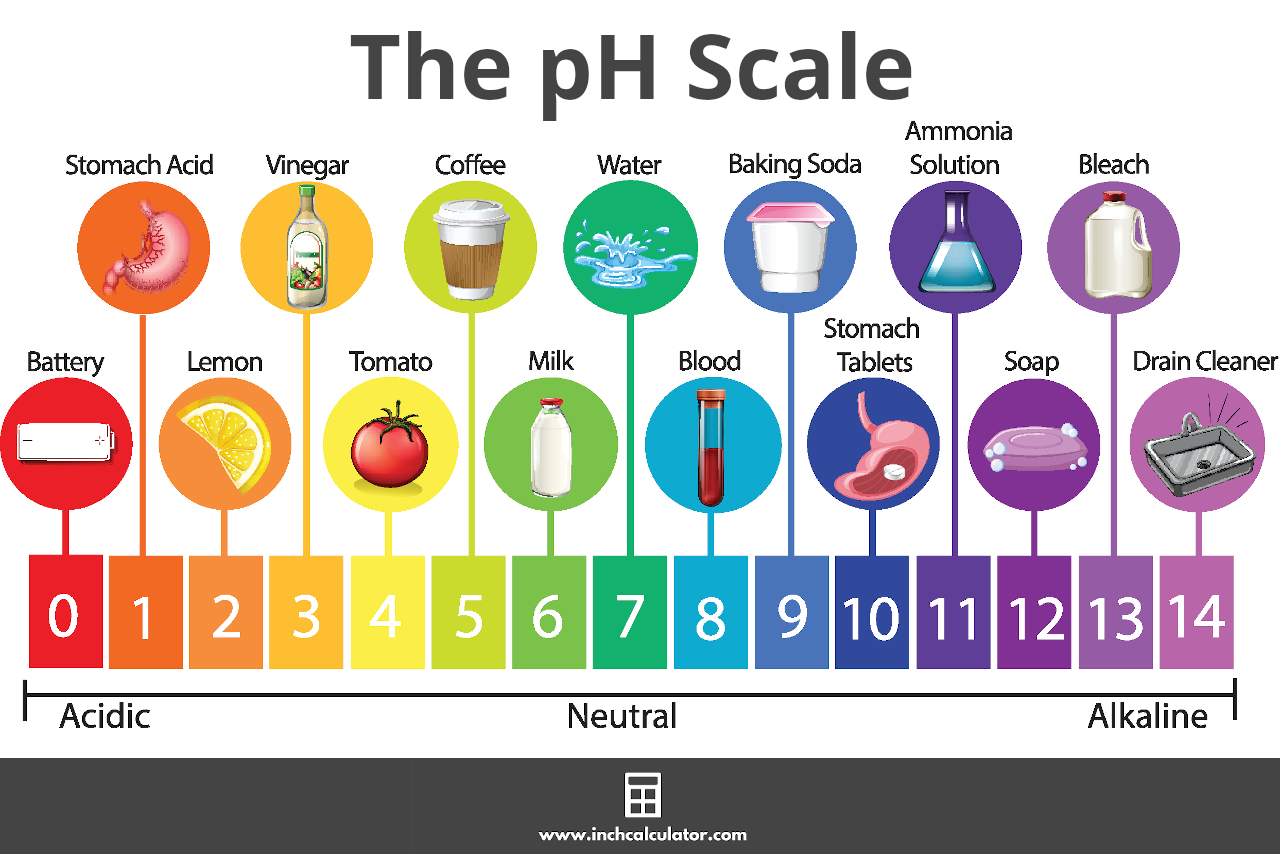

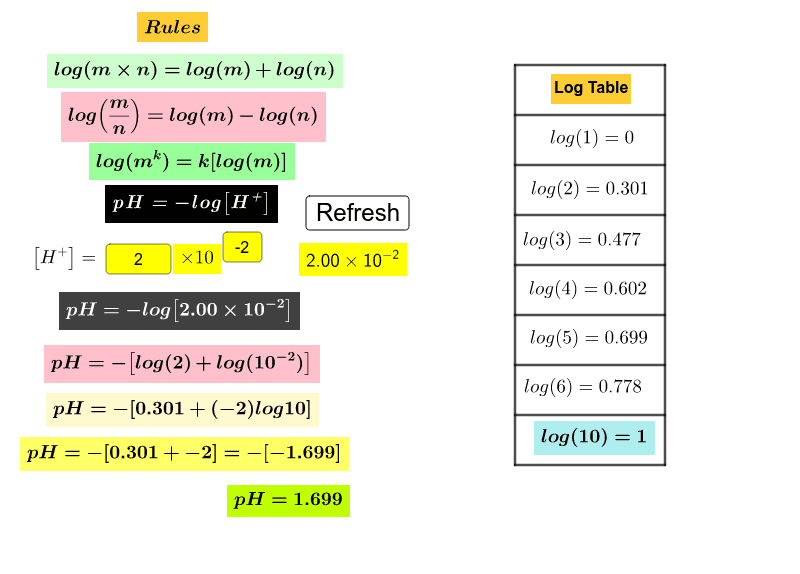
![pH = -log[H+] pH = -log[H+]](https://s3.studylib.net/store/data/008427242_1-6a856521ca6b3bcc442fa00c77f4fe6e.png)


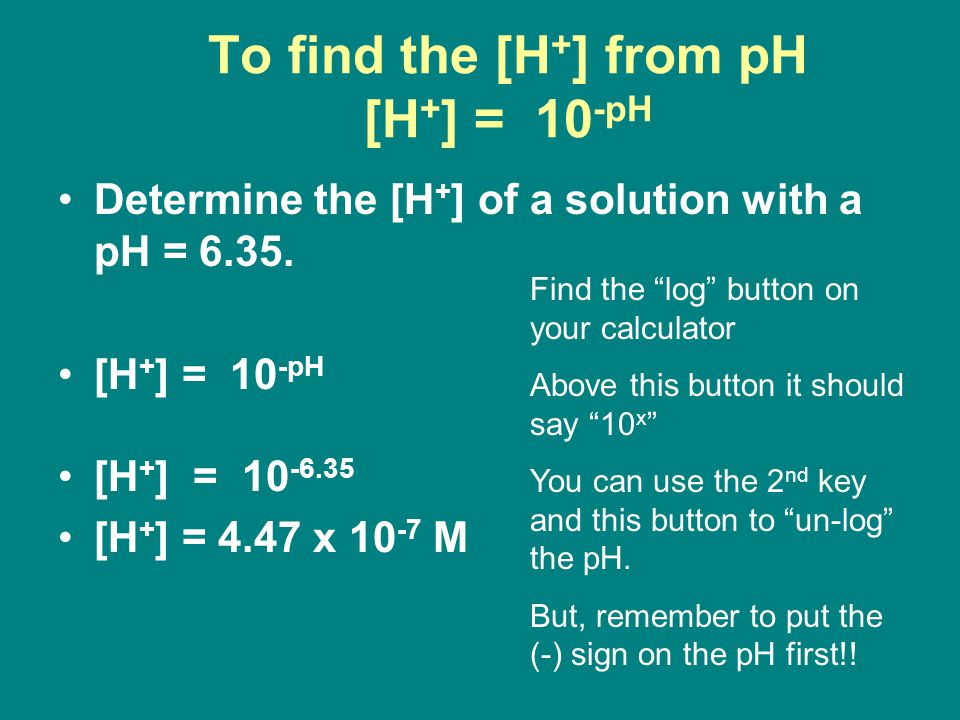
![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)